Enhanced electrocatalytic activity of FeNi alloy quantum dot-decorated cobalt carbonate hydroxide nanosword arrays for effective overall water splitting†
Abstract
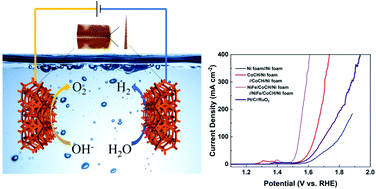
The development of earth-abundant catalysts toward high-efficiency overall water splitting is of critical importance for electrochemical hydrogen production. Here, novel FeNi alloy quantum dot (QD)-decorated cobalt carbonate hydroxide (CoCH) nanosword arrays were successfully constructed on Ni foam (FeNi/CoCH/Ni foam) and used as an efficient bifunctional electrocatalyst for overall water splitting in alkaline media. Benefiting from the synergistic effect between the FeNi alloy QDs and CoCH, the FeNi/CoCH/Ni foam electrode delivers a current density of 20 mA cm−2 at an overpotential of 240 mV and a small Tafel slope of 44.8 mV dec−1 for the oxygen evolution reaction (OER). Further, it displays excellent performance for overall water splitting with a voltage of 1.49 V at 10 mA cm−2 and maintains its activity for at least 23 h. In particular, it only needs low cell voltages of 1.54 and 1.6 V to drive high current densities of 100 and 400 mA cm−2, respectively, which is much better than commercial Pt/C/Ni foam‖RuO2/Ni foam, providing great potential for large-scale application.


 Please wait while we load your content...
Please wait while we load your content...