Chiral carbon dots – a functional domain for tyrosinase Cu active site modulation via remote target interaction†
Abstract
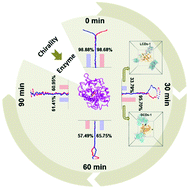
The nano-hybrid enzyme is an ideal catalytic system that integrates various advantages from biocatalysis and nanocatalysis into homogeneous and heterogeneous catalysis. However, great efforts are still needed to fully understand the interactions between nanoparticles and enzymes. Here, we show chiral carbon dots (CDs) as a new functional domain for tyrosinase Cu active site modulation via remote target interaction. Three kinds of chiral CDs (LCDs-1/-2/-3; DCDs-1/-2/-3) were fabricated by thermal treatment of citric acid and L/D-aspartic acid. Then a series of CDs/tyrosinase composites (namely, nano-hybrid-enzymes) were prepared, demonstrating good regulation of enzyme catalytic kinetics. Especially, we find that LCDs-1 is an irreversible inhibitor with great inhibition effect while the others are all reversible inhibitors. Furthermore, it is suggested by both experiments and all-atom molecular dynamics simulations that the joint effect of LCDs-1 and tyrosinase makes LCDs-1 serve as a new functional domain, which has a distinguished ability to control the conformational changes of the key sites of the active center of the tyrosinase (e.g., H60) and thus the escaping behavior of copper ions and the catalytic activity. This work opens a new route for nano-hybrid-enzyme design and enzyme activity regulation with chiral carbon materials as functional domains via remote target interaction.


 Please wait while we load your content...
Please wait while we load your content...