Dual agonist immunostimulatory nanoparticles combine with PD1 blockade for curative neoadjuvant immunotherapy of aggressive cancers‡
Abstract
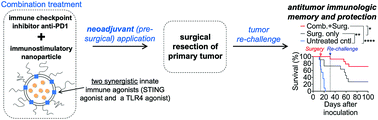
Lethal cancer is characterized by drug-resistant relapse and metastasis. Here, we evaluate the efficacy of a neoadjuvant therapeutic strategy prior to surgery that combines the immune checkpoint inhibitor anti-PD1 with a powerful immunostimulatory nanoparticle (immuno-NP). Lipid-based immuno-NPs are uniquely designed to co-encapsulate a STING and TLR4 agonist that are functionally synergistic. Efficacy of neoadjuvant combination immunotherapy was assessed in three aggressive murine tumor models, including B16F10 melanoma and 4T1 and D2.A1 breast cancer. Primary splenocytes treated with dual-agonist immuno-NPs produced a 75-fold increased production of interferon β compared to single-agonist treatments. Systemic delivery facilitated the widespread deposition of immuno-NPs in the perivascular space throughout the tumor mass and their preferential uptake by tumor-resident antigen-presenting cells. Our findings strongly suggested that immuno-NPs, when administered in combination with anti-PD1, harnessed and activated the otherwise “exhausted” CD8+ T cells as key mediators of tumor clearance. Neoadjuvant combination immunotherapy resulted in significant efficacy, curative responses, and protective immunological memory in 71% of good-responding mice bearing B16F10 melanoma tumors and showed similar trends in the two breast cancer models. Finally, this neoadjuvant combination immunotherapy drove the generation of B and T cell de novo epitopes for a comprehensive memory response.
- This article is part of the themed collection: Nanoscale and Nanoscale Horizons: Nanobiotechnology and Nanomedicine


 Please wait while we load your content...
Please wait while we load your content...