Unusually cyclized triterpenoids: occurrence, biosynthesis and chemical synthesis
Abstract
Covering: 2009 to 2021
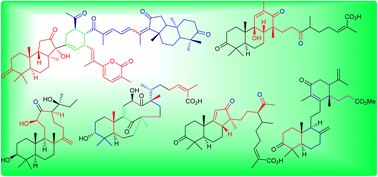
Biosynthetically, most of the syntheses of triterpenes follow the cascade cyclization and rearrangement of the acyclic precursors viz., squalene (S) and 2,3-oxidosqualene (OS), which lead to the very well known tetra- and pentacyclic triterpene skeletons. Aside from these, numerous other triterpenoid molecules are also reported from various natural sources and their structures are derived from “S” and “OS” via some unusual cyclization operations which are different from the usual tetra- and pentacyclic frameworks. Numerous compelling advances have been made and reported in the identification of these unusual cyclized mono-, di-, tri- and tetracyclic triterpenes between 2009 and 2021. Besides a dramatic increase in the newly isolated uncommon cyclized triterpenoids, substantial progress in the (bio)-synthesis of these triterpenes has been published along with significant progress in their biological effects. In this review, 180 new unusual cyclized triterpenoids together with their demonstrated biogenetic pathways, syntheses and biological effects will be categorized and discussed.


 Please wait while we load your content...
Please wait while we load your content...