Visible light-triggered synthesis of oxime ethers using tetrabromomethane as a mediator†
Abstract
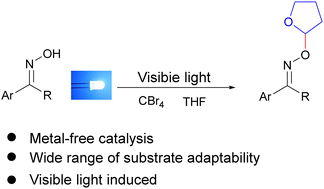
Oxime ethers are of great importance in the field of pharmaceutical chemicals and polymer materials. Herein, we have developed a straightforward synthetic method to prepare oxime ethers starting from tetrahydrofuran or other cyclic ethers and various oxime derivatives with tetrabromomethane as a mediator. A plausible mechanism indicates that tetrabromomethane is first cleaved by visible light irradiation, and the derived radicals transform tetrahydrofuran into its cationic species, which then reacts with oxime to form the target oxime ethers with the formation of a C–O bond. This novel oxidative cross-coupling reaction can be carried out under transition metal-free and mild conditions with good functional group tolerance, and is easy to scale-up.


 Please wait while we load your content...
Please wait while we load your content...