Two new Ni/Co-MOFs as electrocatalysts for the oxygen evolution reaction in alkaline electrolytes†
Abstract
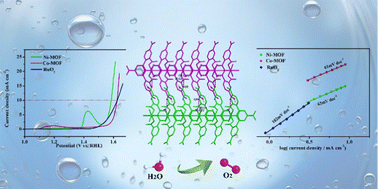
The development of non-noble metal electrocatalysts with excellent performance is very important for the oxidation of water in alkaline media. Metal–organic frameworks (MOFs) have recently emerged as potential catalysts for a variety of reactions due to their atomically dispersed metal centers and accessible active sites. In this work, we report the synthesis of two new Ni-MOF and Co-MOF, [Ni(H2L-NO2)(H2O)3]n (1) and [Co(H2L-NO2)(H2O)3]n (2), as oxygen evolution reaction electrocatalysts. Ni-MOF and Co-MOF demonstrated a good activity with an overpotential of 355 mV and 394 mV to drive a geometrical catalytic current density of 10 mA cm−2 in 1.0 M KOH, transcending the performance of the noble OER catalyst, RuO2. The OER mass activity of Ni-MOF is 1.6 and 3.8 times that of RuO2 and Co-MOF at an overpotential of 350 mV. Moreover, stability tests indicate that these two catalysts demonstrate short-term electrochemical durability.


 Please wait while we load your content...
Please wait while we load your content...