Spectroscopic and theoretical insights into hydrogen bonding clusters, triplet species, and the relaxation mechanism of the light (1ππ*) excited state of 4-methyl-1,2,4-triazole-3-thione†
Abstract
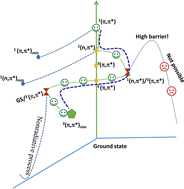
The structures, sizes, reactivities, clusters, and other properties of materials in the microsolvation environment are currently attracting the interest of scientists in materials science and chemical biology. In this study, we investigated the structures of 4-methyl-1,2,4-triazole-3-thione (4MTT) in various environments, including solid, protic, and aprotic solvents. Fourier transform infrared and Raman spectroscopy, and density functional theory calculations were used to assign the vibrational spectra of the 4MTT crystals and microsolvated hydrogen bonding clusters in acetonitrile, water, and methanol. In addition, the ultraviolet-visible (UV-vis) absorption spectra of the microsolvated hydrogen bonding clusters were simulated using time-dependent density functional theory. The UV-vis absorption and Raman spectra of 4MTT results showed that solvent polarity and hydrogen bonding contributed to the observed spectral band shifts in different solvents. The hydrogen-bond networks were centered on the electronegative groups, and the microsolvation clusters were further classified as 4MTT(solvent)n clusters: for n = 1 in acetonitrile and n = 2 in water and methanol. Afterward, nanosecond transient absorption spectroscopy was used to investigate the excited state dynamics in solvents; it was also used in conjunction with complete active space self-consistent field calculations to characterize the excited-state decay mechanism and the formation of long-term triplet species.


 Please wait while we load your content...
Please wait while we load your content...