Carboxylation of terminal alkynes with CO2 catalyzed by imidazolium-bridged bis(phenolato) rare-earth metal complexes†
Abstract
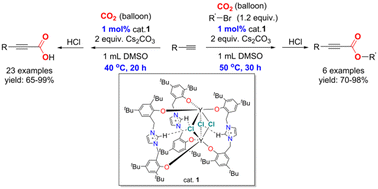
Five imidazolium-bridged bis(phenolato) rare-earth metal complexes 1–5 were synthesized using a readily available bisphenoxy-functionalized imidazolium salt and the corresponding rare-earth metal precursors RE[N(SiMe3)2]3 [RE = Y (1), Yb (2), Sm (3), Nd (4), La(5)], which were well characterized by single crystal diffraction analysis, elemental analysis, and NMR for complexes 1 and 5. The complexes were successfully applied to the carboxylation reactions of terminal alkynes with CO2, where complex 1 showed the highest catalytic activity. A variety of different alkynyl carboxylic acids in excellent yields were obtained under atmospheric pressure. Notably, further esterification occurred smoothly in the current catalytic system via the one-pot three-component reaction using terminal alkynes, CO2 and alkyl bromides as starting materials. Several substituted propargylic esters were obtained in moderate to excellent yields at 1 bar pressure and 50 °C.


 Please wait while we load your content...
Please wait while we load your content...