Product selectivity in the photoreaction of aryl sulfonates and mesylate of estrone derivatives in sustainable and micellar media: a steady-state investigation†
Abstract
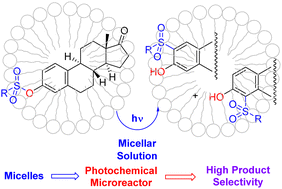
Preparative and mechanistic studies on the photochemical reaction of a series of aryl sulfonates and mesylate of estrone and 17-norestrone derivatives in confined and sustainable micellar environment under steady-state conditions were carried out successfully, and the photochemical behavior was checked against methanol solution. A significant selectivity in favor of the formation of ortho-regioisomers as the main photoproducts over the formation of estrone (or 17-norestrone) was obtained due to the nature of the surfactant, i.e., cationic, anionic, or neutral. Certainly, micelles provide confinement of sulfonic esters within the hydrophobic core, inhibiting the diffusion of the photogenerated radical species into the bulk solution. Because the photoreaction was studied in micellar solutions, the measurement of the binding constant Kb and the estimation of the location of steroids within the hydrophobic core of the micelle were successfully achieved employing UV-visible, 1D-NMR, and 2D-NMR spectroscopic studies. The binding constant values and differential chemical shift (DCS) analysis show the solubilization of sulfonates within the hydrophobic core. In addition, nuclear Overhauser effect spectroscopy (NOESY) experiments clearly demonstrated the location of the sulfonates within the micelles; likewise, diffusion ordered spectroscopy (DOSY) experiments definitively showed that steroids and micelles behave like a unique supramolecular entity.


 Please wait while we load your content...
Please wait while we load your content...