Radical directed regioselective functionalization of diverse alkene derivatives†
Abstract
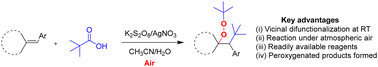
Regioselective vicinal difunctionalization of diverse alkene derivatives was successfully carried out using readily available carboxylic acids. Molecular oxygen in the atmospheric air tethered with the substrate to form the corresponding peroxygenated products in good yields. All the reactions were carried out at room temperature itself. Apart from the synthesis of peroxygenated products, hydroacylated products were obtained by utilizing Csp2 hybridized carbonyl radicals instead of Csp3 hybridized alkyl radicals.


 Please wait while we load your content...
Please wait while we load your content...