ESI-MS reveals preferential complex formation of carbohydrates with Z-sinapinic acid compared with the E-isomer†
Abstract
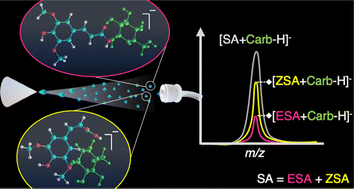
Cinnamic acid derivatives including 3,5-dimethoxy-4-hydroxycinnamic acid, also referred to as sinapinic acid (SA), are efficient matrices used in matrix assisted laser desorption/ionization mass spectrometry (MALDI-MS). Previous findings have shown that Z-sinapinic acid (ZSA) outperforms E-cinnamic acids for carbohydrate (Carb) analysis, probably due to geometry-induced changes in analyte–matrix interactions at the molecular level, prompted by the cinnamic alkene rigid bond. In the present work, direct infusion electrospray ionization mass spectrometry (DI-ESI-MS) experiments were conducted to investigate the behavior of these acids as ligands for neutral Carb non-covalent complex formation. The ionization efficiency and binding affinities of these small molecules were evaluated towards seven Carbs with different monosaccharide composition and length. Experimental results were interpreted with the assistance of computational calculations. ESA, with higher calculated volume and lower acidity than ZSA, exhibited an experimental ESI efficiency similar to the latter. However, titration experiments showed that the [Acid + Carb] gas complex is preferentially formed with ZSA than ESA when both ligands are present in solution. Results from CID experiments support the formation of more stable complexes between larger Carbs and the ZSA isomer compared to the E-form. The change of molecule volume and polarity in the synthesized isotopically labeled ESA, E-3,5-di(methoxy-d3)-4-hydroxycinnamic acid (ESA-d6) used for comparison may explain the lower ESI efficiency obtained with this reference ligand than for ESA. Overall, this combined strategy demonstrated the preferential gas complex formation of Carbs with ZSA, in agreement with the previously proposed matrix–analyte interaction model that explained ZSA differential efficiency as a MALDI matrix.


 Please wait while we load your content...
Please wait while we load your content...