Fabricating 3D ultra-thin N-doped porous graphene-like catalysts based on polymerized amino acid metal chelates as an efficient oxygen electrocatalyst for Zn-air batteries†
Abstract
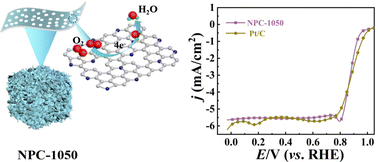
Using metal-free heteroatom-doped carbon material electrocatalysts with high efficiency and low cost for oxygen reduction reaction (ORR) to replace Pt or its alloys is a major task for promoting the reaction efficiency of fuel cells. Here, we report the rational design of three-dimensional (3D) ultra-thin N-doped porous graphene-like carbon (NPC) catalyst based on amino acid zinc chelates containing ligand bonds as both templates and nitrogen sources. During high-temperature annealing treatment, the evaporation of Zn is beneficial to produce a hierarchical porous structure, higher specific surface area and more defect sites. A 3D ultra-thin graphene-like covalently crosslinked polymer with a multilayer network structure was formed by a condensation reaction of amino acid Zn complexes. Under the optimized pyrolysis temperature, the as-obtained NPC-1050 catalyst has a higher defect density and graphitic-N/pyridinic-N ratio, and exhibits an excellent ORR catalytic performance. The half-wave potential, onset potential and limiting current density are 0.878 mV, 0.970 mV and 5.64 mA cm−2, respectively. In addition, it also showed a competitive performance when compared to commercial Pt/C catalysts in Zn-air batteries. The present route may provide new insights into the synthesis of polypeptide or protein metal complexes, and their application in energy fields.


 Please wait while we load your content...
Please wait while we load your content...