Photo-induced charge separation Z-scheme mechanism in ternary bismuth sulfide coupled SnS/Al2O3 nanostructure
Abstract
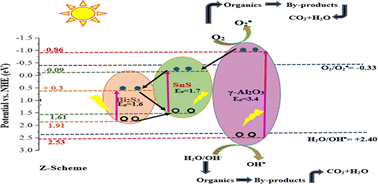
The establishment of a solid-state Z-scheme heterojunction photocatalytic system to efficiently tailor photoinduced charge separation is of great significance to water purification. A structure of metal sulfide nanoparticles, tin monosulfide (SnS) and bismuth sulfide (Bi2S3) in host alumina (Al2O3) was developed by the sol–gel method. FTIR confirmed complete dispersion of SnS and Bi2S3 nanoparticles in Al2O3 having a cubic structure. The band gap energy (Eg) of the ternary nanocomposite was found to be 1.85, 1.68 and 1.10 eV for 4.0, 8.0 and 12.0% bismuth sulfide coupled SnS/Al2O3 respectively. The marked decrease in Eg can be attributed to the tenacious agglomeration among SnS, Bi2S3 and Al2O3. 99.0% methyl orange (MO) removal was achieved with 12.0% bismuth sulfide coupled SnS/Al2O3 due to efficient and quick charge transfer among Al2O3 and chalcogenides via Z-scheme electron transfer mechanism. Electrochemical measurements such as those of linear sweep voltammetry and cyclic voltammetry manifested that bismuth sulfide coupled SnS/Al2O3 exhibited several times more current density than pristine Al2O3 which is very well corroborated with the photocatalytic analysis. Investigation of radical scavengers manifested that O2˙− and OH˙ have important roles in the degradation of MO. This innovative and uniquely designed hybrid ternary nanocomposite will open new horizons as an economical and effective material for photocatalysis.


 Please wait while we load your content...
Please wait while we load your content...