DFT studies on rhodium(iii)-catalyzed synthesis of indanones from N-methoxybenzamides via C–H activation reaction†
Abstract
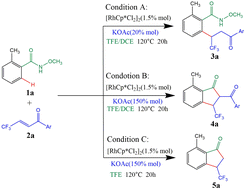
Studies have found that rhodium(III)-catalyzed synthesis of indanones from N-methoxybenzamides with β-trifluoromethyl-α,β-unsaturated ketones via C–H activation reaction is an efficient and direct route. In our study, the mechanism of the Rh(III)-catalyzed C–H annulation of N-methoxybenzamides and β-trifluoromethyl-α,β-unsaturated ketones to form 3-trifluoromethyl-7-methyl-1-indanones is proposed. The results show that the reaction involves N–H deprotonation, C–H activation, insertion of β-trifluoromethyl-α,β-unsaturated ketones into the Rh–C bond, protonation of the seven-membered rhodacycle intermediate, catalyst regeneration, Claisen condensation (including C–N bond cleavage to afford 2-acyl-3-trifluoromethylindanone products), and retro-Claisen condensation producing the 3-trifluoromethyl-7-methyl-1-indanone products. Importantly, the formation of different products under different conditions and the necessary conditions for the formation of 3-trifluoromethyl-7-methyl-1-indanone products are discussed in detail. It is explained why 1,2-dichloroethane cannot carry out the retro-Claisen condensation reaction by calculating the electrostatic potential of 2,2,2-trifluoroethanol and 1,2-dichloroethane. It is concluded that 3-trifluoromethyl-7-methyl-1-indanones need to be synthesized under the conditions of high temperature, high concentration of alkali and 2,2,2-trifluoroethanol. The Claisen condensation and retro-Claisen condensation are the correct paths to form the 3-trifluoromethy-7-methyl-1-lindanone products.


 Please wait while we load your content...
Please wait while we load your content...