Effects of Cu(i) contents on the voltammetric behavior and electrodeposition mechanism of bimetallic composite ionic liquids†
Abstract
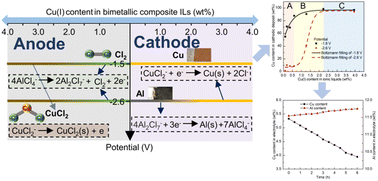
The effective electrochemical recovery of metal for bimetallic composite ionic liquid (IL) is related to the ion species and even the reaction mechanism under different Cu(I) contents. Thus, bimetallic composite ILs (Et3NHCl–1.54AlCl3–xCuCl, x = 0–0.23) were synthesized to investigate the Cu(I) speciation, voltammetric behavior and electrodeposition mechanism under different Cu(I) contents. For the cyclic voltammogram, three cathodic peaks of Et3NHCl–1.54AlCl3–0.010CuCl were related to the reduction of Cu(II) to Cu(I), Cu(I) to Cu(0), and Al(III) to Al(0), respectively. Meanwhile, for Et3NHCl–1.54AlCl3–0.23CuCl, the cathodic and anodic peaks were related to the redox reaction of copper. The whole offline detection of bimetallic composite ILs showed that the anodic reaction under low content of Cu(I) (≤ 0.60 wt%) was dominated by the generation of chlorine gas, while the cathodic reaction was related to the potential. However, when the Cu(I) content was higher than 2.23 wt%, only the disproportionation reaction of Cu(I) occurred and CuCl2 was obtained on the anode. Long-term electrodeposition showed that the effective recovery of copper from industrial bimetallic composite IL was realized. This study lays a good foundation for the clear understanding and comprehensive implementation of the resource utilization.


 Please wait while we load your content...
Please wait while we load your content...