A potent candidate against Zika virus infection: Synthesis, bioactivity, radiolabeling and biodistribution studies†
Abstract
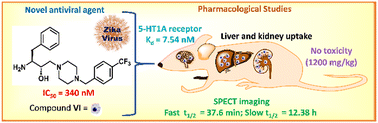
The lack of licensed vaccines and effective drugs against Zika virus (ZIKV) disease creates alarming situations for public health and therefore warrants the discovery of therapeutics. Hydroxyethylamine (HEA) analogs have entered clinical trials for their antiviral properties, presenting a validated pharmacophore for the design of novel antiviral treatments. We thus synthesized HEA compounds (VI and VII) and tested them against ZIKV in culture. Compound VI showed 72-fold higher efficacy to block the infectivity of ZIKV infection over the positive control, 6-methylmercaptopurine riboside. Hit compound VI displayed a 50% inhibitory concentration of 0.34 μM with a selectivity index of 22.47. Biodistribution and bioimaging studies suggested a major uptake of VI in the liver and kidneys of the experimental animals. Slightly lower uptake was also noted in the brain, which showed 6-fold higher accumulation than in the blood. Rhodamine B labeled VI (Rho-VI) was treated with a 5-HT1A receptor that showed a binding affinity of 7.54 nM. Next, compound VI indicated negligible acute and subacute cytotoxicity evaluated in mice. This study supports compound VI as a prime antiviral contender for preclinical and clinical trials against ZIKV disease.


 Please wait while we load your content...
Please wait while we load your content...