Oxidative C–C/C–X coupling in organometallic nickel complexes: insights from DFT†
Abstract
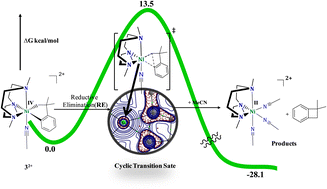
Nickel-based catalysts are employed in organometallic transformations such as C–C/C–heteroatom cross-coupling reactions. The work presented here describes the reactivity of high valent nickel complexes using DFT computations performed at the B3LYP/BS1 level. The calculated bond parameters shed light on how the ligand flexibility changes the reactivity of the nickel centre in various oxidation states of nickel. The reactivity of NiIII-center (2+) and NiIV-center (32+) complexes reveals that direct reductive elimination leading to the C–C coupled product of 1,1-dimethylbenzocyclobutene (P1) is more feasible than the path via five-coordinate intermediates (2+-Int1 and 32+-Int1) as the latter path involves ligand-conformation-change transition states that demand high energy. However, the five-coordinate 32+-Int1 intermediate reacts with MeCN in the sixth position of NiIV-center to generate the 32+-Int2 intermediate that readily undergoes barrierless reductive elimination leading to the product (P1) and this also explains the high yield under blue LED illumination that is observed experimentally. Solvent participation and ligand flexibility are essential for reductive elimination pathways. The Kohn–Sham energy levels of d-orbitals are progressively stabilized when going from the NiII to NiIII to NiIV oxidation states. The HOMO–LUMO gaps (ΔEH–L) show that the overall reactivity decreases in the order 32+ > 2+ > 1. Finally, the reaction of the NiIV-center 32+ complex in the presence of the Cl− nucleophile is investigated. This shows that the reductive elimination of C–C/C–heteroatom coupling can occur through three pathways with almost similar activation barriers (∼18.0 kcal mol−1). This agrees well with the experimental observation that the three products are formed in the reaction almost in the same percentages. QTAIM pictures reveal that the transition-state structures of 32+-TS6, 32+-TS7 and 32+-TS8 relating to reductive elimination from the NiIV-center complex involves a three-membered cyclic transition state.


 Please wait while we load your content...
Please wait while we load your content...