Highly efficient ultralong organic phosphorescence induced by lone pair repulsions and noncovalent interactions†
Abstract
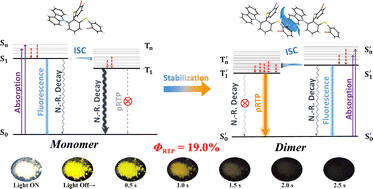
Persistent room temperature phosphorescence (pRTP) has aroused increasing interest. Here we report an ortho-substituted compound in which two o-bromophenylsulfides and a carbazolyl were joined by a phenyl ring. The abundant intra- and inter-molecular noncovalent interactions inhibit the non-radiative transition and make this compound prone to forming a physical dimer which has a stabilized emissive triplet state. The confined space and the repulsion from lone pairs of sulphur atoms rigidize the molecular structure. The compound exhibits a long afterglow of over 2 s with a phosphorescence quantum efficiency (Φp) of 19.0% and a lifetime of over 250 ms. The photoluminescence properties under the aggregational state are also rationalized by computational calculations based on the intermolecular interactions and spin–orbit coupling (SOC) constant.


 Please wait while we load your content...
Please wait while we load your content...