Metal- and base-free regioselective cascade sulfonylation-cyclization of 1,5-dienes via the insertion of sulfur dioxide: access to pyrrolinones†
Abstract
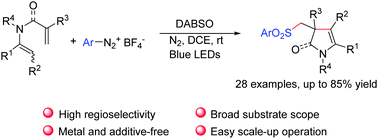
A metal- and base-free regioselective cascade sulfonylation–cyclization reaction of linear substrates, 1,5-dienes, has been developed to synthesize sulfonylated pyrrolinones. This method features mild reaction conditions and offers an alternative route to the class of nonbenzene-fused cyclic molecules containing a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond.
C bond.


 Please wait while we load your content...
Please wait while we load your content...