Strategic design of a 2,6-disubstituted pyridine-based probe having hard-soft centers: responsive divergence from one core†
Abstract
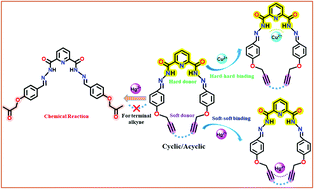
Alkyne is a versatile functional group in organic chemistry, and is able to undergo a wide variety of reactions and interactions. Featuring a reactive functional group, alkyne participates in many organic reactions and hence a sharp rise in the interest of utilizing the alkyne functionality has made it an inevitable synthon in a wide domain of purposes and one of such purposes is molecular recognition. On account of this, two pyridine-derived scaffolds, 5 and 7, containing identical molecular cores but different appendages, viz., terminal alkyne (5) and internally 1,3-conjugated alkyne units (7), are successfully synthesized. Both the compounds are subjected to metal ion sensing at the molecular level and are found to bind Cu2+ and Hg2+ ions with different functionalities. Compounds 5 and 7 interact with Cu2+ by the pyridine N and the two adjacent amide N's in a tripodal fashion, whereas they interact with Hg2+ by their respective open-end and closed-end alkyne units. The terminal alkynes in 5 undergo chemical reaction in the presence of Hg(ClO4)2·H2O and get converted to a ketone functionality, while the internally conjugated 1,3-dialkyne unit in 7 acts as a binding unit for a Hg2+ ion. Both experimental studies and theoretical (DFT) calculations have converged on the result that terminal alkynes cannot function as a chemosensor for Hg2+ ions, although they can respond by functional group transformation, whereas cyclic internally conjugated alkynes can perform as potential Hg2+ sensors. The combination of Cu2+ and Hg2+ ions has been used to generate a molecular system exhibiting the OR logic operation. The limits of detection (LODs) of Cu2+ ions, obtained for 5 and 7, are 5.5 × 10−7 M and 5.2 × 10−7 M, respectively, and that of the Hg2+ ion for 7 is 4.4 × 10−7 M. The synthesized probes, as well as their complexes, are stable around neutral pH and the probes retain their sensitivity within a temperature window of 25–80 °C. This creates an avenue for differential recognition of multiple heavy metal ions simultaneously with similar molecular motifs.


 Please wait while we load your content...
Please wait while we load your content...