Induced cytotoxicity of peptides by intracellular native chemical ligation†
Abstract
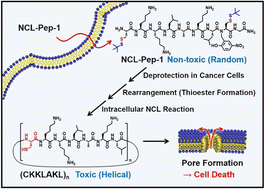
The native chemical ligation (NCL) is a reaction between a pair of peptides, one with a cysteine unit at the N-terminal and the other with a thioester functional group at the C-terminal. The NCL reaction has been widely utilized for the synthesis of various peptides and proteins with long sequences. Here, we demonstrate that the intracellular NCL reaction can endow peptides with induced cytotoxicity in cancer cells. Based on the in situ reduction of protecting groups and spontaneous thioester formation in reducing environment, we hypothesize that the NCL reaction of peptide (NCL-Pep-1) with both N-terminal cysteine and C-terminal crypto-thioester with t-butyl mercaptan protecting groups can occur naturally in cancer cells and can be utilized for the preparation of peptide therapeutic agents. The NCL-Pep-1 peptide produces elongated cyclic peptides via sequential process with deprotection, spontaneous thioester formation, and NCL-induced peptide elongation. Owing to the remarkable enhancement of helicity after elongation, the NCL-Pep-1 peptide induces cancer cell death by intracellular NCL reaction.


 Please wait while we load your content...
Please wait while we load your content...