Insights into the degradation of emerging organic pollutants by peroxydisulfate activated with Co3O4@NiO: role of each component and catalytic mechanism†
Abstract
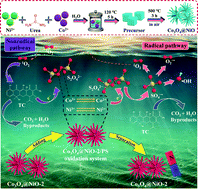
Nowadays, it is a challenging task to achieve the high-efficiency removal of emerging organic pollutants from wastewater. In the current work, a novel Co3O4@NiO-2 composite with great magnetic properties was synthesized by a facile hydrothermal method followed by calcination. Interestingly, the introduction of NiO could improve the specific surface area of Co3O4, while the presence of Co3O4 decreased the charge transfer resistance of NiO. The existence of magnetic properties created more flexibility for the separation and recovery of the catalyst. More importantly, the as-prepared Co3O4@NiO-2 exhibited excellent catalytic performance in PS activation, and the developed Co3O4@NiO-2/PS oxidation system could achieve the effective degradation of various organic contaminants, including TC, BPA, and phenol. During the TC degradation process, radical and nonradical pathways were involved in the PS activation, in which 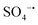 , ˙OH,
, ˙OH, 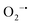 , and 1O2 were the major active species. Furthermore, it was also noted that the Co3+/Co2+ and Ni3+/N2+ redox couples played a crucial role in PS activation for generating radicals, while the direct electron-transfer process among PS, Co3O4@NiO-2, and TCs was involved in TC degradation. In short, in the current study, not only a high-performance catalyst was synthesized for the PS activation, but also some novel insights for further research on the activation mechanism are shared.
, and 1O2 were the major active species. Furthermore, it was also noted that the Co3+/Co2+ and Ni3+/N2+ redox couples played a crucial role in PS activation for generating radicals, while the direct electron-transfer process among PS, Co3O4@NiO-2, and TCs was involved in TC degradation. In short, in the current study, not only a high-performance catalyst was synthesized for the PS activation, but also some novel insights for further research on the activation mechanism are shared.


 Please wait while we load your content...
Please wait while we load your content...