Substituted pyrrolyl-cyanopyridines on the platform of acylethynylpyrroles via their 1 : 2 annulation with acetonitrile under the action of lithium metal†
Abstract
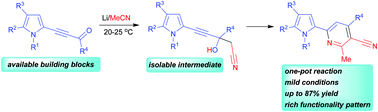
A facile one-pot synthesis of 2-(pyrrolyl)-4-aryl(hetaryl)-5-cyano-6-methylpyridines in 63–87% yield via the cyclization of available acylethynylpyrroles with two molecules of acetonitrile under the action of lithium metal at room temperature has been developed. The reaction proceeds via addition of the −CH2CN anion to the carbonyl group of acylethynylpyrroles followed by intramolecular cyclization of the adduct to the target products.


 Please wait while we load your content...
Please wait while we load your content...