Mapping transition state structures for thiophosphinoyl group transfer between oxyanionic nucleophiles in water and aqueous ethanol solvents†
Abstract
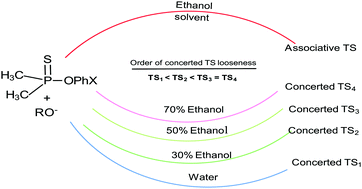
Second-order rate constants were measured for thiophosphinoyl group transfer from substrates 3a-g to oxygen nucleophiles in 50% water–50% ethanol and 30% water–70% ethanol mixtures, to obtain solvent-independent Brønsted coefficients βnuc = 0.35, βlg = −0.64; and βnuc = 0.34, βlg = −0.61, respectively. Combination with earlier results maps a concerted transition state (TS) in water which progressively loosens in 70% water–30% ethanol and 50% water–50% ethanol but remains unchanged thereafter in ethanol-rich mixtures. Analysis with the More O’Ferrall–Jencks reaction map reveals two contending factors: (i) increased nucleophile/leaving group basicity moves the concerted TS towards tighter structures via vector g, while (ii) lowered solvent polarity moves the TS towards dissociative structures via vector z. The inequality z > g in water-rich solvent mixtures leads to more dissociative structures, whilst a parity of these vectors (z ∼ g) in ethanol-rich mixtures leads to an unchanging concerted (dissociative) TS structure. A solvent effect in ethanol-rich solvent mixtures is electrostatic in nature, where the solvent deploys its dielectric endowments to stabilize the significant amounts of charges that develop in the TS. In this work, varying solvent polarity does not lead to a mechanistic change, as previously postulated. The potential energy surface (PES) for reactions involving associative TS structures in ethanol is shown to be markedly different from the PES leading to products via concerted TS structures in water/aqueous ethanol solvents. This finding is of interest in biological phosphoryl transfer systems where it is advocated that enzymes act by tightening the dissociative TS structures of uncatalyzed reactions.


 Please wait while we load your content...
Please wait while we load your content...