Synthesis of sulfamoyl-triazolyl-carboxamides as pharmacological myeloperoxidase inhibitors†
Abstract
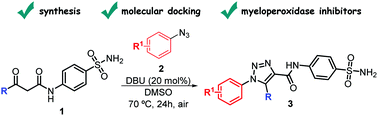
We describe herein the synthesis, molecular docking, protein–ligand interactions and biological validation of 1,2,3-triazolyl-carboxamides containing sulfonamide moieties as anti-inflammatory agents through the inhibition of myeloid hemoprotein myeloperoxidase (MPO) activity. The named compounds were synthesized in good yields under mild conditions, by a [3+2]-cycloaddition reaction of sulfamoyl-β-oxo-amides and aryl azides in the presence of a catalytic amount of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU). Given the higher affinity of some of the synthesized compounds with MPO, their inhibitory activity was evaluated on the plasma of mice as previously reported with a lipopolysaccharide. The obtained results suggest that the sulfamoyl-1,2,3-triazolyl-carboxamides are interesting and promising candidates for further studies on their anti-inflammatory potential.


 Please wait while we load your content...
Please wait while we load your content...