Color-tuning and manipulation of the aggregation-induced emission efficiency of o-carborane-tetraphenylethylene dyads through substituted o-carboranes†
Abstract
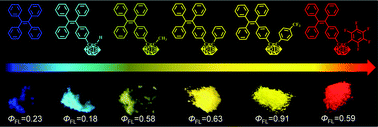
Two new carborane-containing tetraphenylethylene derivatives (1 and 2) were designed and synthesized. Together with our previously published compounds (3–5), we studied structure–activity relationships in detail. The results showed that compounds 1 and 2 exhibited the aggregation-induced emission effect. By changing the 2-R substituents (–H, –CH3, –C6H5, 5F, and CF3) of carborane, absolute solid-state fluorescence quantum yields ranging from 18% to 91% were obtained. The emission colors demonstrated a clear shift from blue to red with a maximum red shift of 239 nm. Quantum calculations were performed in accordance with density functional theory (DFT) and time-dependent DFT to study the excited electronic structures of compounds.


 Please wait while we load your content...
Please wait while we load your content...