XPS as a probe for the bonding nature in metal acetates†
Abstract
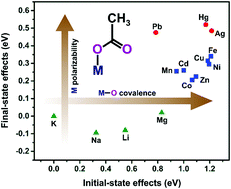
Metal carboxylates are an extensive family of coordination compounds of growing importance in materials science; hence, there is a need for improving the characterization methods for these complexes, especially at the surface level. So far, the use of the XPS technique in this field has been scarce. This work is intended to explore the capabilities of XPS as a reliable probe for the nature of the carboxyl–metal bond. For this, a series of metal acetates was selected as model compounds, covering a wide range of metal cations and coordination geometries. The high-resolution spectra of the C 1s, O 1s, and Auger O KVV lines were recorded and analyzed using curve-fitting procedures. The results indicate that the chemical-state plot for the oxygen atom provides a suitable means for distinguishing metal acetates as a function of the metal cation, whereas the positions of the compounds are not sensitive to the coordination mode. The detected variations in the core-hole extra-atomic relaxation energy can be correlated with the polarizability of the M–O bond due to an admixture of metal states, while the derived variations in the ground-state core eigenvalue depend on the interplay between the electrostatic potential generated by the cation and the degree of ligand-to-metal charge transfer.


 Please wait while we load your content...
Please wait while we load your content...