An understanding of a 3D hierarchically porous carbon modified electrode based on finite element modeling†
Abstract
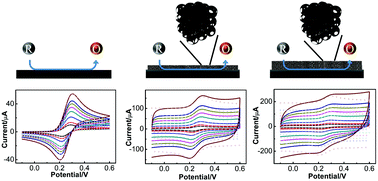
A comprehensive understanding of both redox reactions and electrical double layer capacitance is crucial in the development of electrochemical energy conversion and storage technologies. Herein, a 3D hierarchically porous carbon modified electrode is investigated by both experimental and theoretical cyclic voltammetry based on finite element modeling. An electrochemical reaction rate constant of 1.06 × 10−4 m s−1 is initially obtained based on the proposed model via the reversibility analysis on cyclic voltammograms. Then the redox reaction of ferrocyanide on an FeN hierarchically porous carbon (FeN-HPC) modified electrode is carried out with this rate constant. A comparison of voltammetric waveforms indicates that the redox behavior of ferrocyanide is independent of the electrical double layer capacitance which stemmed from the porous structure and morphology of FeN-HPC. Correspondingly, a specific capacitance of 68.80 F g−1 is obtained with a mass loading of 4 μg on the substrate. Moreover, a comparison of simulated ferrocyanide and ferricyanide concentrations against both applied potential and the distance to the electrode surface further verifies the independence of the redox reaction from the electrochemical capacitance. In summary, this study facilitates the understanding of the redox reaction on a 3D hierarchically porous carbon modified electrode, which is important in the development of energy-related electrode fabrication.


 Please wait while we load your content...
Please wait while we load your content...