Synergetic effects of photocatalysis and peroxymonosulfate activated by FeWO4 for enhanced photocatalytic activity under visible light irradiation†
Abstract
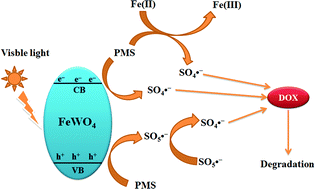
FeWO4 was synthesized by a simple solvothermal method. The band structure of FeWO4 was investigated on the basis of density functional theory calculations. The FeWO4 photocatalyst showed lower photocatalytic activity for the degradation of doxycycline hydrochloride under visible light irradiation. Kinetic studies using radical scavenger technologies suggested that hydroxyl radicals were the dominant photo-oxidants. In order to enhance the photocatalytic activity of FeWO4, FeWO4 was used to activate peroxymonosulfate to degrade doxycycline hydrochloride under visible light irradiation. The influences of the peroxymonosulfate dosage, doxycycline hydrochloride concentration, inorganic anions, natural organic matter and pH value on activating peroxymonosulfate to degrade doxycycline hydrochloride were investigated in detail. The mechanism of FeWO4 activating peroxymonosulfate was proposed and proved by radical quenching experiments, electron spin resonance analysis, X-ray photoelectron spectroscopy, electrochemical impedance spectroscopy, transient photocurrent responses and cyclic voltammetry. It can be found that the combined activation effects of photo-generated electrons and transition metals on peroxymonosulfate to produce sulfate radicals could enhance the degradation efficiency obviously.


 Please wait while we load your content...
Please wait while we load your content...