Using the site-knockout strategy to understand the low activity of the nitrate electro-reduction reaction on Pt(111)†
Abstract
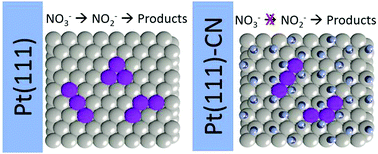
Nitrate and nitrite reduction reactions (NO3RR and NO2RR, respectively) are important processes in water treatment as well as model processes in surface science. The sluggish kinetics observed for NO3RR on Pt electrodes is usually explained by the difficulty in removing NOad. However, NO2RR shares this same intermediate and depicts higher activity under the same experimental conditions. Herein, we employed the site block strategy to show that the nitrate requires contiguous Pt sites to be converted into nitrite and then to NOad. Both NO3RR and NO2RR were studied on Pt(111) and Pt(111) modified with cyanide ions (Pt(111)-CN). While NO2RR depicted lower activity on Pt(111)-CN than on Pt(111), NO3RR was completely inhibited. Regardless of the presence of cyanide, the DFT-based analysis revealed that both NO3 and NO2 adsorption could occur on the bidentate form. However, after this step, extra contiguous sites should be provided to ensure NO3ad proceeds with the reduction reaction, which are not available on Pt(111)-CN. These results bring experimental evidence that nitrate to nitrite conversion is an important bottleneck in NO3RR, and the presence of NOad (produced as intermediate during the NO3RR) does not favor this step once NOad also limits the adsorption site size.


 Please wait while we load your content...
Please wait while we load your content...