Copper–cobalt coordination polymers and catalytic applications on borrowing hydrogen reactions†
Abstract
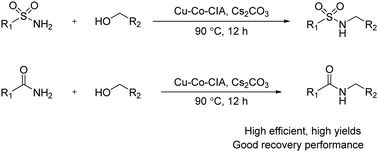
Porous copper and cobalt polymers were designed, synthesized, and characterized by scanning electron microscopy (SEM), energy dispersive spectrometry (EDS), X-ray photoelectron spectrometry (XPS), Fourier-transform infrared (FT-IR) spectroscopy, and X-ray diffraction (XRD). The resulting copper/cobalt composites exhibited a much higher catalytic activity towards the borrowing hydrogen reactions of benzenesulfonamides with benzyl alcohols and carboxamides with alcohols than copper polymers. In addition, copper/cobalt composites could be easily recovered and reused at least six times. Mechanism investigations were conducted to study Cu/Co composites and these transformations.


 Please wait while we load your content...
Please wait while we load your content...