Highly electrochemically and thermally stable donor–π–acceptor triphenylamine-based hole-transporting homopolymers via oxidative polymerization†
Abstract
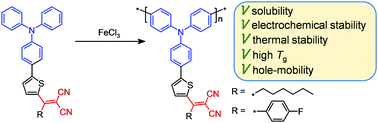
Development of organic semiconducting polymers combining simple and scalable synthesis with high stability, solubility and hole mobility is in high demand. In this work, we report two novel D–π–A triphenylamine-based homopolymers having thiophene as a side π-spacer linked to either hexyl- or 4-fluorophenyldicyanovinyl electron-withdrawing groups. The homopolymers can be easily synthesized via oxidative polymerization at room temperature of the corresponding monomers in the presence of FeCl3 as an oxidant. The polymers possess a number of valuable properties for application in organic and hybrid optoelectronics, among which are high electrochemical and thermal stability (Td is up to 594 °C; coke yield up to 90%), good solubility (up to 90 g L−1 in chloroform), high glass transition temperature (up to 223 °C), efficient light absorption in the UV-Vis region, low-lying HOMO energy levels (ca. 5.36 eV) and sufficient hole mobility (up to 7 × 10−5 cm2 V−1 s−1) without any posttreatment in thin films.


 Please wait while we load your content...
Please wait while we load your content...