Zinc(ii) Schiff base complexes as dual probes for the detection of NH4+ and HPO42− ions†
Abstract
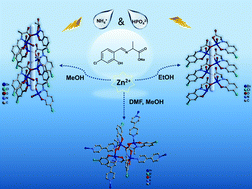
In this paper, a novel Schiff base ligand NaHL (NaHL = sodium (S,E)-2-((4-chloro-2-hydroxybenzylidene) amino) propanoate) was designed and synthesized, and three novel complexes with Zn(II) were obtained by a solvent evaporation technique. Single crystal X-ray diffraction indicated that [Zn(L)(H2O)]n1 and [Zn(L)(H2O)]n2 were one-dimensional coordination polymers (CPs) with a helix structure, while [Zn2(L)2(bipy)] 3 was a two-dimensional CP with the participation of an auxiliary ligand. Fluorescence spectra exhibited that 1 and 2 had good fluorescence properties in both solution and solid states, but 3 was basically quenched due to the addition of ancillary ligands in the solid state. Density functional theory (DFT) calculations have been performed to further reveal the intrinsic fluorescence properties and mechanisms of 1 and 2, which demonstrated the chelate fluorescence enhancement effect (CHEF). Moreover, 1 and 2 show selective recognition of NH4+ and HPO42− accompanied by an efficient fluorescence “turn off” phenomenon, and a series of related experiments demonstrate that 1 and 2 were fully quenched through the removal of Zn2+ from the complex and the protonation of the ligand.


 Please wait while we load your content...
Please wait while we load your content...