Design, synthesis and in vitro evaluation of the hybrids of oxindolylidene and imidazothiazolotriazine as efficient antiproliferative agents†
Abstract
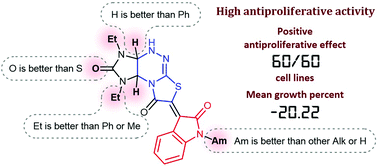
1,3-Diethyl-6-oxindolylidenetetrahydroimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine-2,7-dione with a 2-propyl substituent at the nitrogen atom of the oxindole fragment (1d) was identified previously as a lead compound in an effort to discover antiproliferative agents based on oxindolylidene derivatives of imidazothiazolotriazine. A broadened structural optimization using an earlier developed efficient synthetic route provided 17 new oxindolylidene imidazothiazolotriazines which displayed evident antiproliferative activity in cellular assays (GI50 0.60–8.37 μM). The most potent compounds 5d–s (GI50 < 4.5 μM) contained the 1,3-diethyltetrahydroimidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine core and an alkyl substituent at the nitrogen atom of the oxindole fragment. Compound 5m with an amyl substituent at the nitrogen atom of the oxindole fragment possessed higher antiproliferative activity with mean growth of 60 cancer cell lines −20.22%, and average values of GI50 1.45 and TGI 3.43 μM. Compound 5m was not toxic against the normal Madin–Darby canine kidney MDCK-M cell line (IC50 > 100 μM). The IC50 value of compound 5m against normal human embryonic kidney cells HEK293 was 17.65 μM, which appeared to be 3-fold higher than the IC50 value of 5m against rhabdomyosarcoma cells.


 Please wait while we load your content...
Please wait while we load your content...