New pyrimidine-5-carbonitrile derivatives as EGFR inhibitors with anticancer and apoptotic activities: design, molecular modeling and synthesis†
Abstract
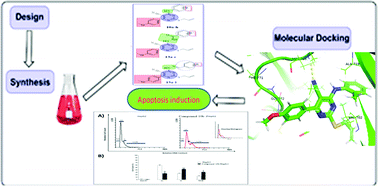
In connection with our efforts in the development of new anticancer agents, herein we report the design and synthesis of new small pyrimidine-5-carbonitrile based derivatives. The target pyrimidines were evaluated in vitro for their anticancer activity against three cancer cell lines: hepatocellular carcinoma (HepG2), non-small cell lung cancer (A549) and breast cancer (MCF-7) cell lines. Compounds 10a, 10b, 13a, 13b, 15a, 15e and 15j exhibited the highest activities towards the three cell lines. In particular, compound 10b exhibited excellent activities against HepG2, A549 and MCF-7 cell lines with IC50 values of 3.56, 5.85 and 7.68 μM, respectively, compared to erlotinib as a reference drug with IC50 values of 0.87, 1.12 and 5.27 μM, respectively. Additionally, the effect of the most cytotoxic derivatives on EGFR inhibition was assessed. Compound 10b emerged as the most potent EGFR inhibitor with an IC50 value of 8.29 ± 0.04 nM, in comparison with that of erlotinib (IC50 = 2.83 ± 0.05 nM). Moreover, compound 10b arrested the cell growth in HepG2 cells at the G2/M phase and induced a significant increase in apoptotic cells. Finally, the binding patterns of the target derivatives were investigated by a docking study against the proposed molecular target (EGFR, PDB ID: 1M17).


 Please wait while we load your content...
Please wait while we load your content...