Insight into the dehydration of high-concentration fructose to 5-hydroxymethylfurfural in oxygen-containing polar aprotic solvents†
Abstract
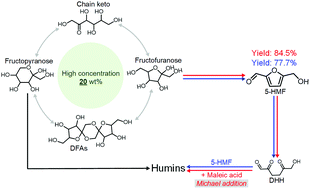
In this study, 1,4-dioxane and tetrahydrofuran are found to be the most suitable solvents for the dehydration of fructose at high concentrations, in which 77.7% and 78.8% yields of 5-hydromethylfurfural (5-HMF) are obtained, respectively, from 20 wt% fructose in the presence of an acid catalyst (5 mol% HCl, 11 wt% water). The high proportion of fructofuranose formed in those polar aprotic solvents and the reversible dimerization to the protective difructose dianhydrides determined by 13C NMR and HPLC-MS, which stop fructose from excess cross-polymerization into unwanted humins, are considered to be the key factors for the satisfactory 5-HMF yield. Further addition of strong electrophilic molecules such as 5 mol% maleic acid, which consumes 2,5-dioxo-6-hydroxy-hexanal and blocks its reaction with 5-HMF by Michael addition, enhances the yield of 5-HMF to 85.4%.


 Please wait while we load your content...
Please wait while we load your content...