Self-assembly driven chiral transfer from a dipeptide to the twist and stacking handedness of cyanobiphenylyl groups†
Abstract
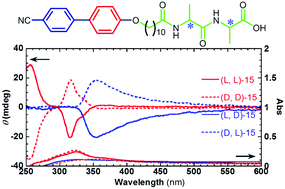
The cyanobiphenylyl group was linked to the terminal alkyl chain of Ala–Ala lipodipeptides with different chiralities. The lipodipeptides can self-assemble into twisted nanoribbons in a mixture of methanol and water. Fourier transform infrared spectra indicated that H-bonding among the amide groups and the hydrophobic association of alkyl chains played important roles in the formation of nanoribbons. The peptide chains were packed in parallel β-sheet structures. It was found that the twist and stacking handedness of cyanobiphenylyl was controlled within the nanoribbons. On the basis of optical and morphological characterization, the twist handedness of cyanobiphenylyl groups is controlled by the chirality of alanine near the alkyl chain, while the handedness of nanoribbons and the stacking handedness of cyanobiphenylyl groups are determined by the chirality of alanine at the C-terminal. Moreover, the X-ray diffraction patterns indicated that homogeneous and heterogeneous lipodipeptides were packed in different structures.


 Please wait while we load your content...
Please wait while we load your content...