Anti-cancer activity of heteroaromatic acetals of andrographolide and its isomers†
Abstract
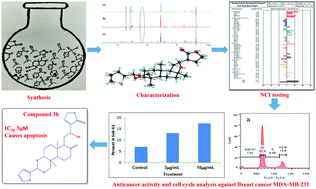
Acetals (2a–d, 3a–d, and 6a–d) of andrographolide (1), 14-deoxy-12-hydroxyandrographolide (4), and isoandrographolide (5) were synthesized using benzaldehyde and heteroaromatic aldehydes. All the synthesized derivatives were characterized using 1H-NMR, 13C-NMR, mass spectrometry, UV, and IR. The compound 6d was characterized via a single-crystal X-ray diffraction study. All the compounds were tested against 60 cell lines of NCI. The acetals (2a–d) of andrographolide (1) exhibited better activity than the acetals (3a–d, and 6a–d) of 12-hydroxyandrographolide (4) and isoandrographolide (5). Preliminary studies suggested that acetals synthesized using benzaldehyde improved anticancer activity. Compound 2a showed the highest growth inhibition of 90.97% against the leukaemia cancer cell line CCRF-CEM. Andrographolide and seven selected compounds were tested against the MDA-MB-231 breast cancer cell line. Compound 3b showed the best activity with an IC50 value of 3 μM among all the tested compounds. Furthermore, this compound 3b was subjected to cell cycle analysis and protein expression confirming apoptosis through the disruption of the mitochondrial potential membrane (Δψm).


 Please wait while we load your content...
Please wait while we load your content...