An α-naphtholphthalein-derived colorimetric fluorescent chemoprobe for the portable and visualized monitoring of Hg2+ by the hydrolysis mechanism†
Abstract
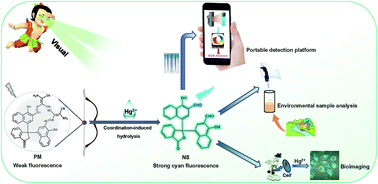
A novel fluorescent probe, 3,3′-(((1E,1′E)-((3-oxo-1,3-dihydroisobenzofuran-1,1-diyl)bis(1-hydroxynaphthalene-4,2-diyl))bis(methaneylylidene))bis(azaneylylidene))bis(2-aminomaleonitrile) (PM), which consists of an α-naphtholphthalein scaffold and dual diaminomaleonitrile moieties, was designed and successfully synthesized by Duff and Schiff-base condensation reactions. PM can be employed for colorimetric fluorescent detection of Hg2+ in aqueous THF solution. The detection limit of PM for Hg2+ was calculated to be 20 nM. Noteworthily, the sensing mechanism of PM for Hg2+ involves metal ion-induced hydrolysis of the diaminomaleonitrile-based Schiff-base skeleton, which has been confirmed by reversibility experiments, UV-Vis, HRMS, and 1H NMR titrations approaches, as well as DFT calculations. Due to its excellent fluorimetric properties, a portable smartphone-assisted intelligent platform was established to realize the convenient, cost-effective, and reliable detection of Hg2+. Significantly, the in-field detection of environmental water samples was achieved with good recoveries through the portable platform. In addition, PM was successfully applied to monitor the intracellular Hg2+ ions in HeLa cells. More importantly, the strategy developed here is of benefit towards the design of excellent specificity chemoprobes for Hg2+ detection in sulfur-rich living organisms.


 Please wait while we load your content...
Please wait while we load your content...