Comparative study of BiVO4 and BiVO4/Ag2O regarding their properties and photocatalytic degradation mechanism†
Abstract
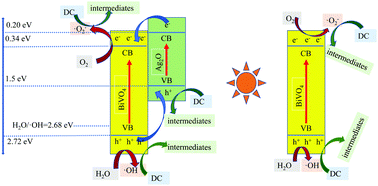
BiVO4, as a visible light catalyst, produces photogenerated electrons and photogenerated holes that are easy to recombine. In this paper, a BiVO4/Ag2O composite was prepared to promote the separation of photogenerated carriers of BiVO4. Compared with BiVO4, the properties and photocatalytic activity of BiVO4/Ag2O were obviously improved. The improvement of BiVO4/Ag2O was reflected in the better separation of photogenerated electrons and photogenerated holes, higher UV-visible maximum absorption wavelength, and stronger ability to degrade doxycycline. The effects of Ag2O on the crystal structure, the morphology and the binding energy of BiVO4 were analyzed by XRD, XPS and SEM, and the existence of a heterojunction in BiVO4/Ag2O was confirmed. The results of capture experiments showed that the photocatalytic processes of BiVO4 and BiVO4/Ag2O produced hydroxyl radicals (˙OH), holes (h+) and superoxide radicals (˙O2−). However, the contribution of active species was different in the two systems. The degradation pathways and intermediate products of doxycycline were analyzed by UPLC-QTOF. This work proposed a new method for the preparation of BiVO4/Ag2O particles with remarkable photocatalytic activity, and provided a reference for the degradation of doxycycline.


 Please wait while we load your content...
Please wait while we load your content...