An orthogonal approach for the precise synthesis of phenylpropanoid sucrose esters†
Abstract
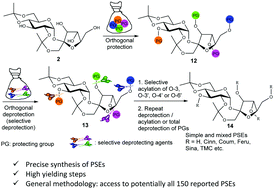
Phenylpropanoid sucrose esters (PSEs) are plant-derived metabolites that exist widely in medicinal plants and possess important bioactivities. Their precise synthesis is challenging due to the distinct and diverse substitution patterns at the sugar framework, and it is scarcely reported. Orthogonal protection/deprotection strategies for disaccharides are more complex and less developed than those for monosaccharides. We disclose a precise synthesis of PSEs starting from 2,1′:4,6-di-O-diisopropylidene sucrose 7via an orthogonal protection/deprotection and selective cinnamoylation strategy. We demonstrate the strategy for the synthesis of several PSEs cinnamoylated at the O-3 and O-4′ positions of diisopropylidene sucrose 7. The strategy is enabled by a carefully selected and synergistic set of protecting groups and deprotecting agents under the optimized conditions. It potentially gives access to the ∼150 reported PSEs and opens the door for the custom synthesis of unnatural PSEs for industrial applications. The reported work also presents a viable strategy for the general orthogonal protection/deprotection of disaccharides for the precise synthesis of other classes of phenylpropanoid esters and related compounds.


 Please wait while we load your content...
Please wait while we load your content...