Improvement of quasi-solid-state supercapacitors based on a “water-in-salt” hydrogel electrolyte by introducing redox-active ionic liquid and carbon nanotubes†
Abstract
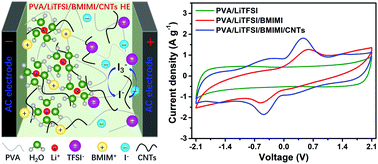
In comparison with supercapacitors (SCs) with aqueous solution electrolytes, quasi-solid-state SCs (QSCs) based on hydrogel electrolytes (HEs) exhibit more extensive application prospects due to the advantages of, for example, easier encapsulation and deformability. The increment in specific energy (SE) of QSCs has become a prevailing research topic. In this work, a “water-in-salt” (WiS) HE with a wide electrochemical window and redox nature was prepared by mixing polyvinyl alcohol (PVA) and bis(trifluoromethane sulfonyl)imide (LiTFSI, 10 m (mol kgwater−1)) WiS solution, and then adding a dispersion composed of ionic liquid (IL) 1-butyl-3-methylimidazolium iodide (BMIMI) along with highly conductive carbon nanotubes (CNTs) to the PVA/LiTFSI HE, which is named PVA/LiTFSI/BMIMI/CNTs HE. By optimizing the amounts of IL BMIMI and CNTs, the PVA/LiTFSI/BMIMI/CNTs HE possesses remarkable ionic conductivity (47.5 mS cm−1). A QSC with the sandwich-type configuration containing the optimized PVA/LiTFSI/BMIMI/CNTs HE together with two activated carbon (AC) electrodes was assembled. The constructed QSC device displays a wide working voltage (2.1 V) and can deliver a high specific capacity (292.2 F g−1) as well as a large SE (40.6 W h kg−1) at 0.5 A g−1. The prominent energy storage capability for this quasi-solid-state device should be attributed to the wide working voltage due to the retention of the nature of the WiS electrolyte, the occurrence of I−/I3− redox reactions at the AC electrode/HE interface contributing the extra pseudocapacitance and the production of conductive network as a result of CNTs added in the HE. Moreover, this device possesses fascinating long-term cyclic stability and its self-discharge is suppressed to some extent due to the addition of CNTs.


 Please wait while we load your content...
Please wait while we load your content...