Pluronic F127-assisted partial decomposition of ammonium phosphomolybdate and its application as an efficient and recyclable catalyst for the alkylation of toluene with benzyl alcohol†
Abstract
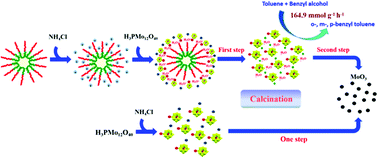
Acidic ammonium phosphomolybdate was prepared under the assistance of Pluronic F127 and applied as the catalyst for the alkylation of toluene with benzyl alcohol. F127 contributed to the assembly of heteropoly anions with counter-ions in a hydrothermal process via the interaction between heteropoly anions and the protonated EO blocks of F127. Ammonium ions and the hydronium ions generated by protonation could be uniformly distributed around heteropoly anions. The removal of F127 made the decomposition and oxidation of ammonium ions occur at lower temperatures before the decomposition of heteropoly anions. Protons were formed due to the decomposition of ammonium ions, thereby leading to the formation of acidic ammonium phosphomolybdate solid solution. Under an optimum amount of F127, the obtained catalyst showed excellent catalytic performance in the alkylation of toluene with benzyl alcohol with an activity of 164.9 mmol g−1 h−1. More importantly, this catalyst was found to have good stability and recyclability in liquid-phase reactions.


 Please wait while we load your content...
Please wait while we load your content...