Peptide probes with high affinity to target protein selection by phage display and characterization using biophysical approaches†
Abstract
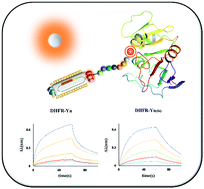
Polypeptides provide unique possibilities for the development of efficient and selective fluorescent probes for different biomarkers because of their highly modular nature, the feasibility of chemical synthesis and fluorescence modification, and biomolecular recognition potential. Herein, a bacteriophage (phage) display was utilized to screen affinity peptides against dihydrofolate reductase. On this basis, positive peptide DHFR-Y1(NH2-WSLGYTG-COOH) was obtained utilizing solid-phase peptide synthesis. Furthermore, fluorescein FAM was used to chemically modify the N-terminus and C-terminus of the peptide, respectively, to generate N-terminal fluorescent peptide DHFR-Yn (Flu-βA-WSLGYTG-COOH) and C-terminal fluorescent peptide DHFR-Yc (NH2-WSLGYTL(Flu)-COOH). In the subsequent experiment, multiple in vitro detection methods (including DSF, STD-NMR, BLI) indicated that the binding ability of affinity peptide DHFR-Y1 (KD = 3.57 μM) is significant to DHFR, in contrast to the stronger noncovalent binding ability of C-terminal fluorescent peptide (KD = 0.56 μM) and weak affinity of N-terminal fluorescent peptide (KD = 14.70 μM). The evidence that DHFR-Y1 and DHFR-Yc bind to folic acid binding pocket of DHFR was coming up by the in silico study. The peptide DHFR-Yc is adequate as a fluorescent probe targeting dihydrofolate reductase.


 Please wait while we load your content...
Please wait while we load your content...