NiOOH@Cobalt copper carbonate hydroxide nanorods as bifunctional electrocatalysts for highly efficient water and hydrazine oxidation†
Abstract
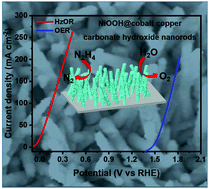
Replacing the sluggish oxygen evolution reaction (OER) with the hydrazine oxidation reaction (HzOR) has been proved to be an efficient approach to increase the hydrogen production efficiency through water electrolysis. Herein, NiOOH@cobalt copper carbonate hydroxide (NiOOH@CoCu CH) nanorods were designed for highly efficient water and hydrazine oxidation. Compared with NiOOH and CoCu CH directly grown on nickel foam, NiOOH@CoCu CH demonstrates enhanced electrocatalytic activities toward the OER and HzOR. To achieve a current density of 10 mA cm−2, the required potentials for the OER and HzOR are 1.49 V and −0.031 V vs. reversible hydrogen electrode (RHE), respectively. Also, it just needs an overpotential of −171 mV to acquire a current density of 10 mA cm−2 during the hydrogen evolution reaction (HER) process. By assembling a two-electrode electrolyzer using NiOOH@CoCu CH as both the anode and cathode in an alkaline electrolyte with hydrazine, a rather low cell voltage of 0.087 V is required to realize a current density of 10 mA cm−2. This value is far smaller than that of 1.56 V required in pure KOH electrolyte, suggesting a more energy-saving approach for efficient hydrogen production. Moreover, NiOOH@CoCu CH also demonstrates excellent long-term operation stability during continuous OER, HER and HzOR processes. All these merits corroborate the promising practical applications of such non-noble metal-based electrocatalysts for large scale hydrogen production through water electrolysis with the assistance of the hydrazine oxidation reaction.


 Please wait while we load your content...
Please wait while we load your content...