Characterization of interactions of montelukast sodium with human serum albumin: multi-spectroscopic techniques and computer simulation studies
Abstract
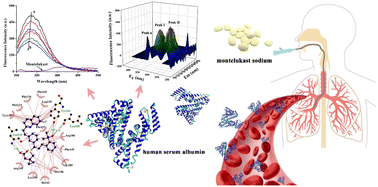
Montelukast sodium (MLS) is a leukotriene receptor antagonist that is widely used to treat asthma in clinical treatment. To further understand the pharmacological mechanism of MLS in the human body, detailed studies on its physiological effects at the molecular level are important. In this study, the mechanism of interaction between MLS and human serum albumin (HSA) was investigated using a multispectral technique and computational simulations. Fluorescence spectroscopy experiments and UV-vis absorption results clarified that MLS quenched the inherent fluorescence HSA through a static quenching mechanism. The binding was a spontaneous process driven by hydrophobic forces and hydrogen bonds. Three-dimensional, synchronous fluorescence spectroscopy, FT-IR and circular dichroism spectroscopy confirmed that MLS altered the amino acid microenvironment and conformation of HSA. Competition experiments revealed that the MLS bound to HSA in the hydrophobic cavity of the IB domain. Docking and molecular dynamics simulation was further carried out and showed the binding conformation, in which the binding was stable and reduced the flexibility and ductility of HSA. The binding of MLS also influenced the surface hydrophobicity of HSA. This work provides important information for investigating the interaction between MLS and HSA to further explore the transport mechanism of MLS in the blood. These results contribute to the development of leukotriene receptor antagonists and the application of MLS.


 Please wait while we load your content...
Please wait while we load your content...