Investigation of the interactions between three flavonoids and human serum albumin by isothermal titration calorimetry, spectroscopy, and molecular docking
Abstract
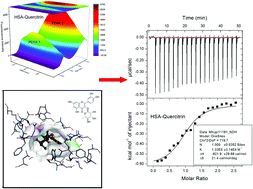
The interactions between three flavonoids (liquiritin, quercitrin, and taxifolin) and human serum albumin (HSA) are investigated by spectroscopic techniques, isothermal titration calorimetry (ITC), and molecular docking study. The results show that the binding of flavonoids to HSA is a static quenching process. Both ITC and fluorescence results reveal that the interactions between HSA and flavonoids are driven by entropy change. Quercitrin is the strongest quencher for HSA because of the enthalpy change. The changes in the conformation of HSA are observed by the addition of flavonoids. Molecular docking results are consistent with the results of thermodynamic experiments. This work provides clues for elucidation of the mechanism of the interactions between flavonoids and HSA.


 Please wait while we load your content...
Please wait while we load your content...