Conversion of scrap iron into ultrafine α-Fe2O3 nanorods for the efficient visible light photodegradation of ciprofloxacin†
Abstract
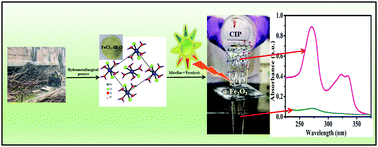
The present study illustrates a feasible approach of utilizing scrap iron for the synthesis of iron(II) oxide. The as-obtained product was further utilized for the degradation of the pharmaceutical pollutant ciprofloxacin (CIP). Iron was recovered from scrap metal wire via a hydrometallurgical process. In this process, the iron wire was converted to highly pure ferrous chloride crystals. The pure ferrous chloride crystals were then used as an iron source for the stabilization of iron oxalate nanorods via a micellar route. The as-obtained iron oxalate nanorods were further calcined at 250 °C and 450 °C to obtain iron oxide nanorods (4 nm × 10 nm) and nanoparticles (25 nm), respectively. The iron oxide nanorods and nanoparticles were then used for the efficient photodegradation of CIP. The iron oxide formed via calcination at 250 °C had a reasonably good surface area of 133 m2 g−1. Investigations to gain detailed mechanistic insight into the Fe2O3 nanorods for CIP degradation indicated that e− and ˙OH were the major reactive species involved in the degradation of CIP. The photocatalyst showed good durability and recyclability for over three consecutive reaction cycles. Moreover, the present study demonstrates a plausible route for the conversion of scrap iron into a useful product serving the dual advantages of mitigating its disposal problems as well as the remediation of waste water.


 Please wait while we load your content...
Please wait while we load your content...