Efficient one-step synthesis of 3-(indol-2-yl)quinoxalin-2(1H)-ones via electrochemical oxidative cross-dehydrogenative coupling†
Abstract
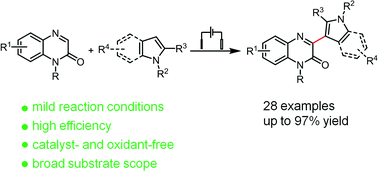
Developing an efficient and convenient method for direct synthesis of bioactive 3-(indol-2-yl)quinoxalin-2(1H)-ones is highly desirable in the pharmaceutical industry. In this work, 3-(indol-2-yl)quinoxalin-2(1H)-ones were synthesized by a one-step electrochemical cross-dehydrogenative coupling process from quinoxalin-2(1H)-ones and indoles. This protocol is simple, operationally convenient, and compatible with a broad range of substrates, enabling the synthesis of the desired coupling products in good to excellent yields (up to 97%) without the use of any catalyst or chemical oxidant. In addition, a reaction mechanism based on the electrochemical oxidation of indoles was proposed.


 Please wait while we load your content...
Please wait while we load your content...