Mechanism of halide exchange in reactions of CpRu(PPh3)2Cl with haloalkanes†
Abstract
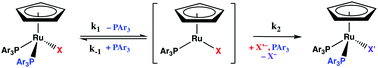
Kinetic measurements of the reaction between CpRu(PPh3)2Cl (1a) and 1-bromobutane reveal a nearly first order dependence on the concentration of haloalkane and a negative entropy of activation, ΔS† < 0. The rate of halide exchange (kobs = 0.56 ± 0.03 × 10−6 s−1 at 35 °C) is two orders of magnitude lower than the rate of phosphine dissociation (kobs = 47.1 ± 1.1 × 10−6 s−1 at 35 °C). The reaction rate decreases in the presence of excess PPh3 and when 2-bromo-2-methylpropane is substituted for nC4H9Br. The rate dependence on [nC4H9Br] is consistent with a two term rate law for the reaction: rate = kobs[1] = (k1 + k2[RBr])[1]. A mechanism where formation of CpRu(PPh3)(RBr)Cl in a second order reaction (k2 = 1.2 ± 0.1 × 10−8 M−1 s−1) is slightly lower rate than a subsequent first order reaction leading to the final product (k1 = 4.6 ± 3.1 × 10−7 s−1) is proposed. DFT calculations are consistent with either oxidative addition or σ-bond metathesis as lower energy pathways than single electron transfer and formation of a radical pair. The reaction between CpRu(PAr3)2Cl (PAr3 = PPh3, PPh2(p-tolyl), P(p-tolyl)3, P(p-FC6H4)3 and P(p-CH3OC6H4)3, 1) and haloalkanes also provides an efficient route for the synthesis of the corresponding bromides and iodides CpRu(PAr3)2Br (2) and CpRu(PAr3)2I (3).


 Please wait while we load your content...
Please wait while we load your content...